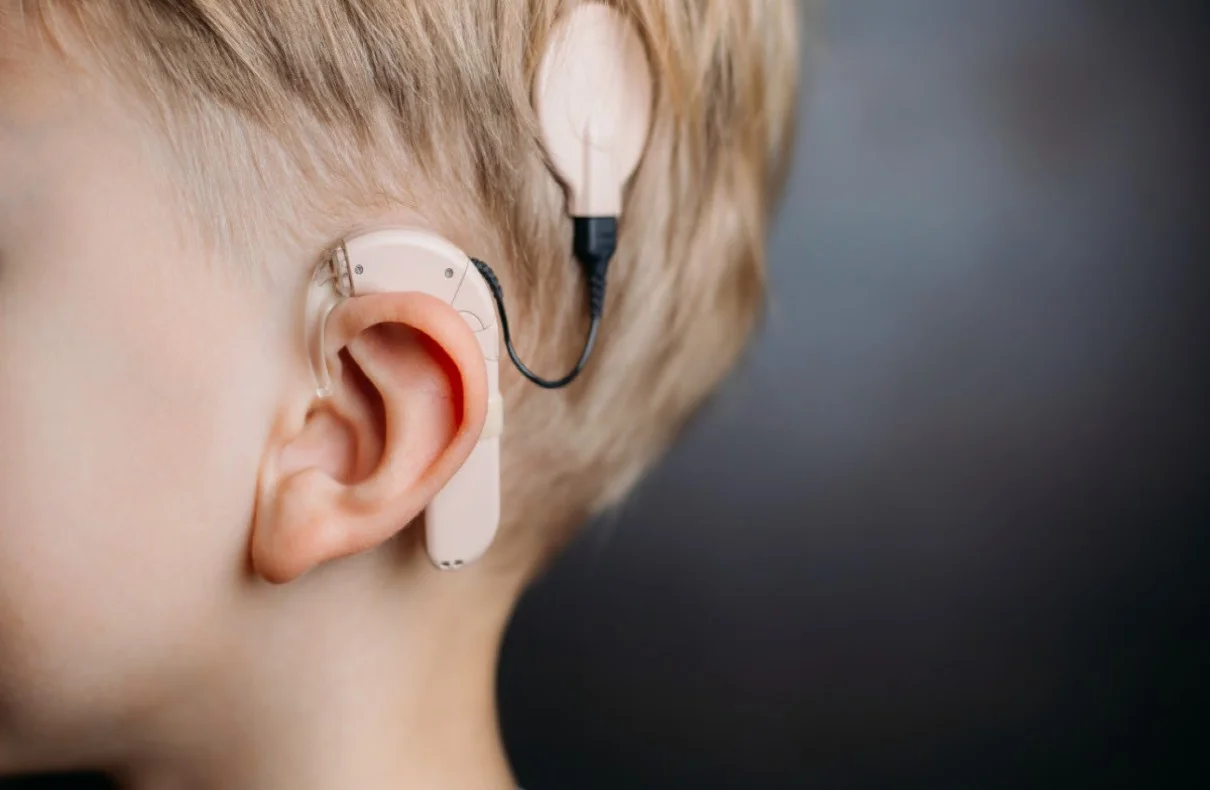
Hearing loss is a prevalent sensory condition that affects millions of people worldwide. Despite its prevalence, there are currently no approved pharmacologic treatments for this condition. However, recent advancements in gene therapy have brought a glimmer of hope to individuals with hearing loss. In an extraordinary breakthrough, Eli Lilly & Co., a leading pharmaceutical company, has successfully restored the hearing of a child born deaf using an experimental gene therapy called AK-OTOF. This groundbreaking therapy targets the otoferlin gene mutations responsible for a specific form of hearing loss.
AK-OTOF is a novel gene therapy developed by Eli Lilly & Co. and acquired through their subsidiary, Akouos Inc. This therapy utilizes a dual adeno-associated viral vector-based gene transfer approach to treat hearing loss caused by mutations in the otoferlin gene (OTOF). Otoferlin is a protein crucial for the proper functioning of inner hair cells in the cochlea, and mutations in this gene are associated with profound hearing loss. AK-OTOF aims to restore hearing by delivering a healthy version of the otoferlin gene to the inner ear, enabling the production of functional otoferlin protein.
FDA Approves Revolutionary Cellular Therapy for Metastatic Melanoma
In a remarkable clinical trial, an 11-year-old child with profound hearing loss from birth became the first individual in the United States to receive the AK-OTOF gene therapy. Within just 30 days of treatment, the child experienced a complete restoration of their hearing. The results were astounding, with hearing restored across all tested frequencies and falling within the normal range at some frequencies. This groundbreaking achievement offers hope to the millions of individuals worldwide who suffer from hearing loss due to otoferlin gene mutations.
The success of the AK-OTOF gene therapy represents a significant milestone in the field of hearing loss treatment. Scientists and physicians have been tirelessly working towards the development of gene therapy for hearing loss for over two decades. The initial results from this trial have exceeded expectations, demonstrating that gene therapy may restore hearing to a degree previously thought impossible. Dr. John Germiller, the principal investigator of the clinical trial, expressed his optimism, stating that these results provide hope for a future where hearing can be restored using genetic medicine.
Individuals with hearing loss caused by otoferlin gene mutations often face profound hearing impairment from birth. Unfortunately, many of them have not undergone the necessary genetic testing to receive a definitive diagnosis. The AK-OTOF gene therapy offers a promising solution for these individuals, as it targets the specific genetic cause of their hearing loss. By delivering a functional otoferlin gene to the inner ear, this therapy has the potential to restore hearing in those affected by OTOF-mediated hearing loss. Approximately 200,000 people worldwide are estimated to have hearing loss related to otoferlin gene mutations, making this breakthrough therapy a ray of hope for a large patient population.
Ensuring the safety and tolerability of any medical intervention is of paramount importance. In the case of the AK-OTOF gene therapy, Eli Lilly & Co. reported no serious adverse events related to the treatment. Both the surgical administration procedure and the investigational therapy were well-tolerated by the study participant. These reassuring findings provide further confidence in the potential of AK-OTOF as a safe and effective treatment for otoferlin gene mutations and associated hearing loss.
Gary Sinise: A Tribute to His Son, McCanna Anthony Sinise
The positive results from the initial trial of AK-OTOF have paved the way for further research and development in the field of gene therapy for hearing loss. Eli Lilly & Co. aims to present additional data from the clinical trial, including initial data from a second participant, at the upcoming Association for Research in Otolaryngology’s meeting in February 2024. This presentation will shed more light on the efficacy and potential of AK-OTOF in restoring hearing. The gene therapy has already been granted orphan drug designation and rare pediatric disease designation by the U.S. Food and Drug Administration, acknowledging its potential to address unmet medical needs in the field of hearing loss.
The successful restoration of hearing in an 11-year-old child using the AK-OTOF gene therapy marks a significant breakthrough in the treatment of hearing loss. This achievement not only offers hope to individuals with otoferlin gene mutations but also paves the way for advancements in gene therapy for other forms of hearing loss. The field of gene therapy for hearing loss has long been a focus of scientists and physicians, and the initial results of this trial demonstrate the tremendous potential of this approach. With further research and development, gene therapy has the potential to revolutionize the treatment of hearing loss and bring the joy of sound to millions of individuals around the world.