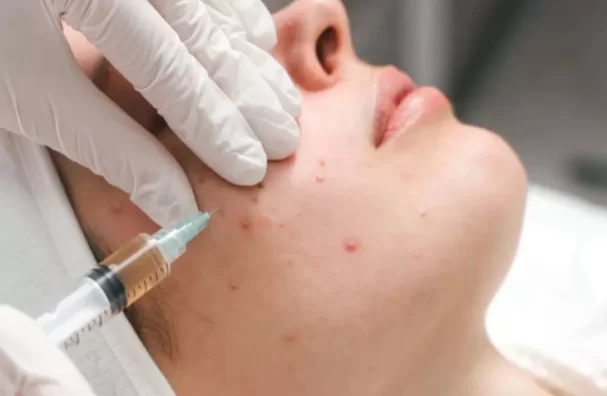
Unregulated cosmetic practices can pose an alarming health risk to consumers, as highlighted by a recent incident involving three women contracting HIV following a “Vampire Facial” procedure at an unlicensed spa.
“Vampire Facial” refers to a popular cosmetic procedure also known as platelet-rich plasma micro needling. During this procedure, the patient’s blood is drawn and spun to separate plasma from blood cells. The plasma, rich in platelets, is then injected back into the patient’s face using microneedles. Despite claims of skin rejuvenation, there is scant scientific evidence supporting the effectiveness of such procedures.
The incident began to unravel when a woman in her 40s received a positive result on a rapid HIV test during a trip abroad in summer 2018. Although she exhibited no clear risk factors for HIV – such as injection drug use, blood transfusions, or multiple sexual partners – she disclosed having undergone a Vampire Facial at a spa called VIP Spa in Albuquerque, New Mexico, earlier in the year.
The woman’s case prompted an investigation into VIP Spa, which was found to be operating without a license. The spa had no appointment scheduling system and did not maintain client contact information. A subsequent inspection revealed shocking conditions at the facility including unwrapped syringes, unlabeled blood tubes, and reused disposable equipment. Moreover, there was no autoclave – a pressurized oven needed for sterilizing equipment – on the premises.
The spa was promptly shut down and its owner charged with practicing medicine without a license. A second woman, who also had a Vampire Facial at VIP Spa, tested positive for HIV later in 2018. Two more cases were identified in 2021 – a woman who had undergone three Vampire Facial procedures at VIP Spa and her male partner. Genetic sequencing showed that the infections were closely related.
The lack of comprehensive client records posed a significant challenge during the investigation, necessitating a large-scale outreach to identify potential cases. Nonetheless, the investigation underscores the importance of identifying possible novel sources of HIV transmission among individuals with no known HIV risk factors.
This incident underscores the potential dangers associated with unregulated cosmetic procedures. Consumers seeking such treatments must take precautions to ensure the safety and legitimacy of the providers. It is also a potent reminder for authorities to enforce strict health and safety regulations in the cosmetic industry.
